
High Potency sweeteners
Sweeteners come from various sources. They have been sought throughout history for their pleasing taste and many uses.The science of sweetness, however, goes beyond the source of the foodstuff for the sweetener. At a molecular level, approximately 100 chemicals are sweet. They all are referred to as sugars.
Sugar substitutes
Sugar substitutes are of three types:
- Intense sweeteners,
- Low calorie sweeteners and
- Baulking sweeteners.
This post will focus on the Intense sweeteners.
Intense sweeteners are also called non-nutritive sweeteners because they are so much sweeter than sugar that the small amounts needed to sweeten foods contribute virtually no calories to the foods. These sweeteners also do not promote tooth decay. Currently, four such intense sweeteners are available, both for use in processed foods and for home consumption.
High Potency sweeteners /Intense sweeteners
1.Acesulfame K
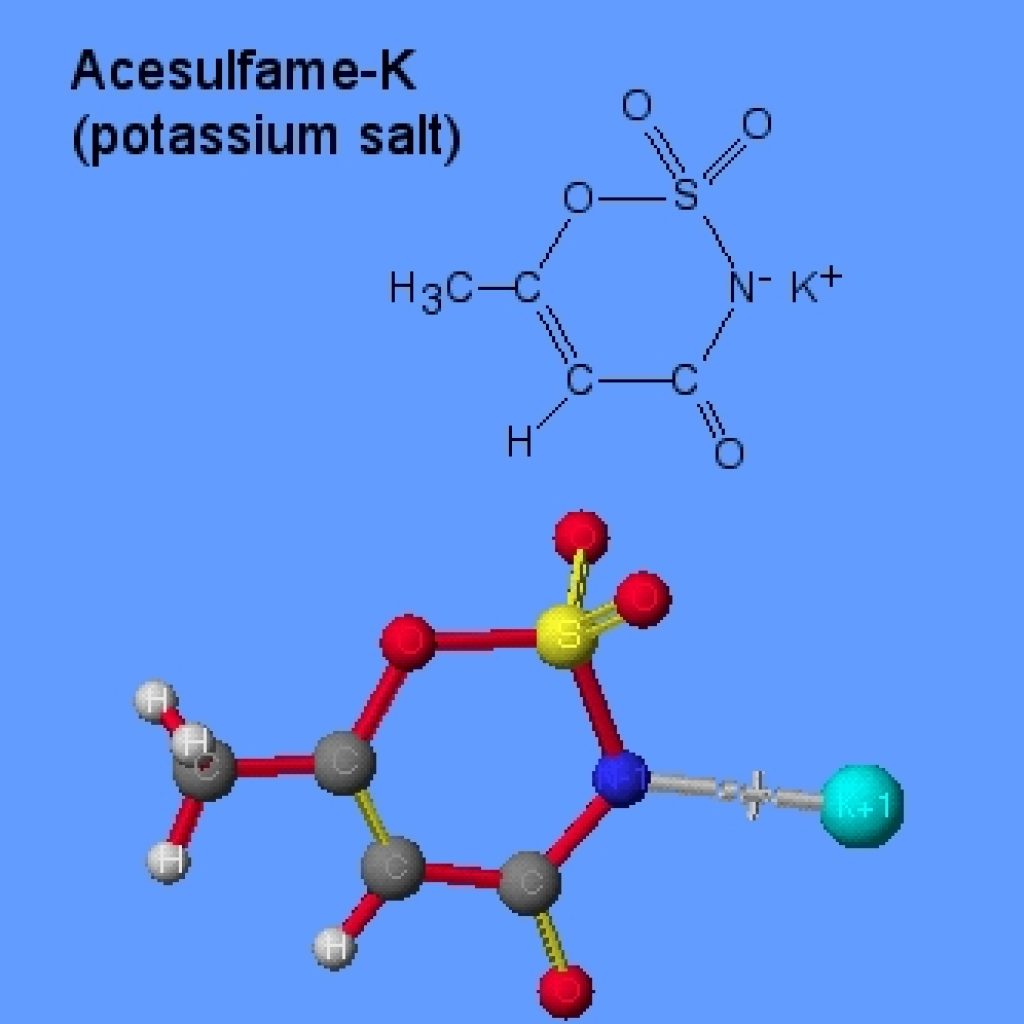
- It was discovered and developed by Hoechst AG and is now marketed worldwide by the former food ingredients business of this company, Nutrinova Nutrition Specialties & Food Ingredients GmbH under the trademark Sunett .
- It is approximately 200 times sweeter than sucrose when used at moderate sweetness levels.
- The sweetness of Acesulfame K is perceived quickly and without any unpleasant delay (especially in comparison with aspartame and sucralose). The sweetness does not, as a rule, linger and normally does not persist longer than the intrinsic taste of the food in which it is used.
- In the human body, Acesulfame K is absorbed, and similarly excreted, rapidly. After a single dose, excretion is virtually complete within 24 hours and it is excreted completely unchanged.in the urine. Therefore, it is non-caloric. No metabolism was observed in humans or other animal species.
- Acesulfame K is not metabolised by bacteria in the oral cavity or in the gut. It cannot therefore be transformed into acids that attack the tooth enamel.
- In the human body, Acesulfame K is inert. No substance-specific effects were found in the screening programme for potential pharmacological effects. These included secretion of insulin and the level of blood glucose.
- An acceptable daily intake (ADI) of 0–15 mg/kg .
- Allocation of an ADI (accepted daily intake )concludes that consumption by children and pregnant women is also acceptable.
- The ADI of Acesulfame K is equivalent to the sweetness of approximately 180 g sugar per day for the 60 kg adult. When blends with other sweeteners are used, this may increase to more than 250 g per day as a result of the synergistic sweetness enhancement of some blends.
- Therefore, sugar equivalents to the ADI of Acesulfame K are much higher than the sugar consumption.
- Direct tableting of Acesulfame K is not easy.
Uses
- On the basis of favourable safety assessments, Acesulfame K is approved worldwide for food use, normally for a wide variety of foods and especially beverages, table-top sweeteners, dairy products and chewing gum.
- The EU approval includes more than 30 product categories.
- In the United States, Acesulfame K is approved as a ‘general purpose sweetener and flavour enhancer’ for use under good manufacturing practice. This means it can be used without formal limits in all non-standardised products and in standardised products when it is listed as such or by general reference to intense sweeteners or sweetening agents in the list of ingredients.
2. ASPARTAME

- Aspartame was discovered by Schlatter in 1965 in the laboratories of G.D. Searle and there followed a period of rigorous testing before it first appeared in the US market in 1981 under the brand name of NutraSweet. The brand was heavily promoted and contributed to the phenomenal commercial success of aspartame as a sucrose replacement in the 1980s and 1990s.
- Aspartame is a white, colourless, crystalline substance, is regarded as ecologically safe and is a biodegradable non-regulated material.
- Aspartame is used in many applications in the food and pharmaceutical industry. Current estimates suggest that it accounts for 32% of the global high-intensity sweetener market and the major markets for the product are soft drinks and table-top sweeteners.
- However, it is also used in confectionery, pharmaceutical tablets and dry syrups, dairy products, dry mix products and bars.
- Aspartame is permitted in all major markets and its safety has been proved at various occasions.
- It is a nutritive intense sweetener produced by combining the amino acids l-phenylalanine and l-aspartic acid by a methyl-ester link.
- Aspartame has a clean sweet taste and has approximately 180–200 times the sweetness of sucrose. Unlike many other intense sweeteners, its taste profile is good enough and its maximum sweetness intensity of 13–14%4 sucrose equivalence (SE), high enough for it to be used as the sole sweetener in most applications.
- Aspartame is synergistic with many bulk and intense sweeteners with the levels of synergy being dependent on concentration and blend constituents. Synergy has been reported with glucose, sucrose, fructose, polyols, saccharin, cyclamate, Acesulfame, K12 and Stevia.
- Aspartame is a nutritive sweetener, in that it is broken down in the body to its two constituents: amino acids and methanol. The amino acid metabolites follow the normal routes of digestion and utilisation in the body as they would if generated from other food sources .
- Aspartic acid makes up approximately 40% of the aspartame molecule. It is absorbed in the intestinal lumen and plays an important role in nitrogen and energy metabolism, in the mitochondria.
- Phenylalanine makes up over half the aspartame molecule. It is an essential amino acid (i.e. the body cannot synthesize it) and it must, therefore, be obtained from foods for normal growth to be maintained. Adults require a minimum of 1–2 g of l-phenylalanine per day.
- The requirement for l-phenylalanine is highest in infants (where growth rate is rapid) and it reduces as growth rate declines.
- Methanol is a potentially harmful metabolite. Various studies have shown that even at abuse levels, the methanol levels produced by aspartame consumption are barely measurable, insignificant and do not represent a health risk.
- One 330 mL can of soft drink sweetened with aspartame at 550 mg/L would, in theory, generate 18.3 mg of methanol – that is approximately 0.26 mg/kg BW methanol in a 70 kg person.
- For comparison, a 220 mL glass of tomato juice would generate 47 mg methanol or 0.67 mg/kg BW in a 70 kg person. The small amount of methanol produced is likewise excreted from the body in an identical fashion to methanol produced from other food sources (e.g. banana, tomato juice).
Other health beneficial effects of Aspartame
- Aspartame is not fermented by tooth plaque bacteria and is to be considered tooth-friendly.
- Aspartame does not affect blood glucose levels and is, therefore, suitable for use in foods for diabetics.
- Overall use of aspartame is an effective way to lose weight. The rate of weight loss that can be achieved is low but meaningful and Impactful.
3. NEOTAME
- It is a derivative of aspartame and is next-generation product to Aspartame. It is a white/off-white crystalline powder.
- Neotame, as it became branded, was a product of a research collaboration started in the mid-1980s between Tinti and Nofre at Claude Barnard University France and The NutraSweet Company.
- Neotame is approximately 8000 times as sweet as sucrose and has a clean sweet taste similar to sucrose.
- It has a liquorice side-taste that is increasingly noticeable, as sweetness tails off and also as its concentration increases. It does not have bitter or metallic side-tastes
Oral Health
- Neotame is not metabolised by oral bacteria and is considered to be non-cariogenic.
- Neotame does not affect glycaemic control in patients with non-insulin-dependent diabetes.
The projected consumption level in the population is estimated to be 0.05 mg/kg BW per day. The no observed effect level (NOEL) for chronic toxicity and carcinogenicity is estimated to be at least 1000 mg/kg BW per day in rats and 800 mg/kg BW per day in dogs.
Both Neotame and its main degradation product,deesterified Neotame, have been shown to be non-mutagenic and well tolerated above projected chronic consumption levels.
Key Uses
- Many products (particularly, soft drinks), use combinations of Neotame and sugar or high fructose glucose syrup (HFGS). It is also used in combination with other intense sweeteners (e.g. saccharin and sucralose).In soft drinks, the use level in a ‘typical’ carbonated soft drink (9–12% sucrose) would be 0.045–0.07%. A 30% sugar reduction on a full sugar product can be obtained using 6.75–9.0% sugar and 0.002–0.003% of Neotame.
- Use of Neotame in chewing gum is usually in combination with other sweeteners, which are often encapsulated. Use of free Neotame in the gum base is claimed to give long-lasting sweetness in gum.
- Other applications that currently utilize Neotame include dairy drinks, ambient sauces, confectionery bars, chewing gum, fresh breath capsules and savoury snacks.
4. ADVANTAME
- While the NutraSweet Company was developing Neotame, Ajinomoto was also looking to develop the next generation of intense sweeteners. This product was developed by Ajinomoto while working in optimizing the Neotame in Japan.
- Advantame is reported to have a RS of 7000–47,000 times that of sucrose over the range 3–14% SE and 70–120 times the sweetness of aspartame over the same range.
- Sweetness quality is described as clean, sweet and similar to aspartame; weak bitter and sour notes are present.
- Flavour enhancement has been found with a number of fruit flavours and vanilla at very low use levels (about 0.005 mg/100 mL.
- Maximum human exposure for general use is estimated at 0.05 mg/kg per day giving.
5. SACCHARIN

- Saccharin , sometimes referred to as o-benzoic sulfimide, was the first commercially developed, sweet-tasting organic compound, which was significantly more potent than sucrose. The discovery of saccharin was reported in 1879 by Remsen and Fahlberg.
- The major applications of saccharin are in beverages, either in finished products such as carbonated soft drinks, or in beverages sweetened with saccharin as a table-top sweetener.
- In these applications, the only requirement is sweet taste and so a second ingredient providing additional sucrose functionality is unnecessary.
- Saccharin is limited, however, by bitter and metallic off-tastes when employed as a sole sweetener and, as a result, it is most commonly employed in blends with other high-potency sweeteners such as aspartame or cyclamate.
- The common caloric sweetener sucrose is used in many applications including baked good, frozen dessert, confectionery, processed food, beverage and table-top applications.
- Saccharin, as well as other high-potency non-caloric sweeteners, however, only find significant usage/in the latter two categories, beverages and table-top applications. In these applications, saccharin provides only sweetness. Other foods require additional physical properties that are provided by sucrose but not saccharin.
- Absorption: Saccharin is largely absorbed from the small intestine. In a human study with radio-labelled saccharin, only 5% of the dose was recovered in the faeces, thus indicating that 95% of the dose is absorbed into the circulation.
- Metabolism: Saccharin is not metabolised in humans .In addition, saccharin does not covalently bind to DNA in the bladder, as would be expected if saccharin is a carcinogen of the classical type .
- For saccharin to bind with DNA, metabolism to an electrophilic species would be necessary and the failure of this to occur is consistent with an absence of carcinogenicity.
- It was associated with cancer in mice when it was fed in very large amounts. However, further studies have found no links between saccharin and human cancer. This recently led the U.S. government to remove it from its list of potential cancer-causing chemicals.
- Excretion: In humans, oral doses are excreted almost completely by the kidneys with the balance recovered in the faeces.
6. SUCRALOSE

- Sucralose is the direct result of an intensive research programme carried out in the 1970s by Tate&Lyle, PLC.
- It is uniquely made from sucrose by a process of chemical modification that results in the enhancement of the sweetness intensity, retention of a pleasant sugar-like taste and creation of a very stable molecule.
- This latter property makes sucralose suitable for use in low pH and neutral products as well as heat-processed foods.
- It is a very versatile sweetener that can be used in a wide variety of foods and beverages, and it allows the development of an increasing range of good-tasting, low-calorie foods, including baked goods.
- Sucralose is approved for use in food products in most countries around the world and has become very popular with both consumers and the food and beverage industry.
- Sucralose is made by chemically modifying sucrose (table sugar) to a non-nutritive, noncaloric powder that is about 600 times sweeter than sugar.
- Before approving sucralose, the FDA reviewed more than 110 research studies conducted in both human and animal subjects. It concluded that the sweetener is safe for consumption by adults, children, and pregnant and breastfeeding women in amounts equivalent to the consumption of about 48 pounds of sugar annually (an Acceptable Daily Intake of 5 milligrams per kilogram of body weight).
- People with diabetes may also safely consume the sweetener, because it is not metabolized like sugar.
- In addition, sucralose is highly stable to heat and so will not lose its sweetness when used in recipes that require prolonged exposure to high temperatures (such as baking) or when stored for long periods.
- The product is currently available in the form of a powdered sugar substitute and in some commercial baked goods, jams and jellies, sweet sauces and syrups, pastry fillings, condiments, processed fruits, fruit juice drinks, and beverages, and its use is approved for various additional products. However, use of sucralose in home baking is expected to be limited by its low bulk in comparison with table sugar.